Contract
Development
Manufacturer
Organisation
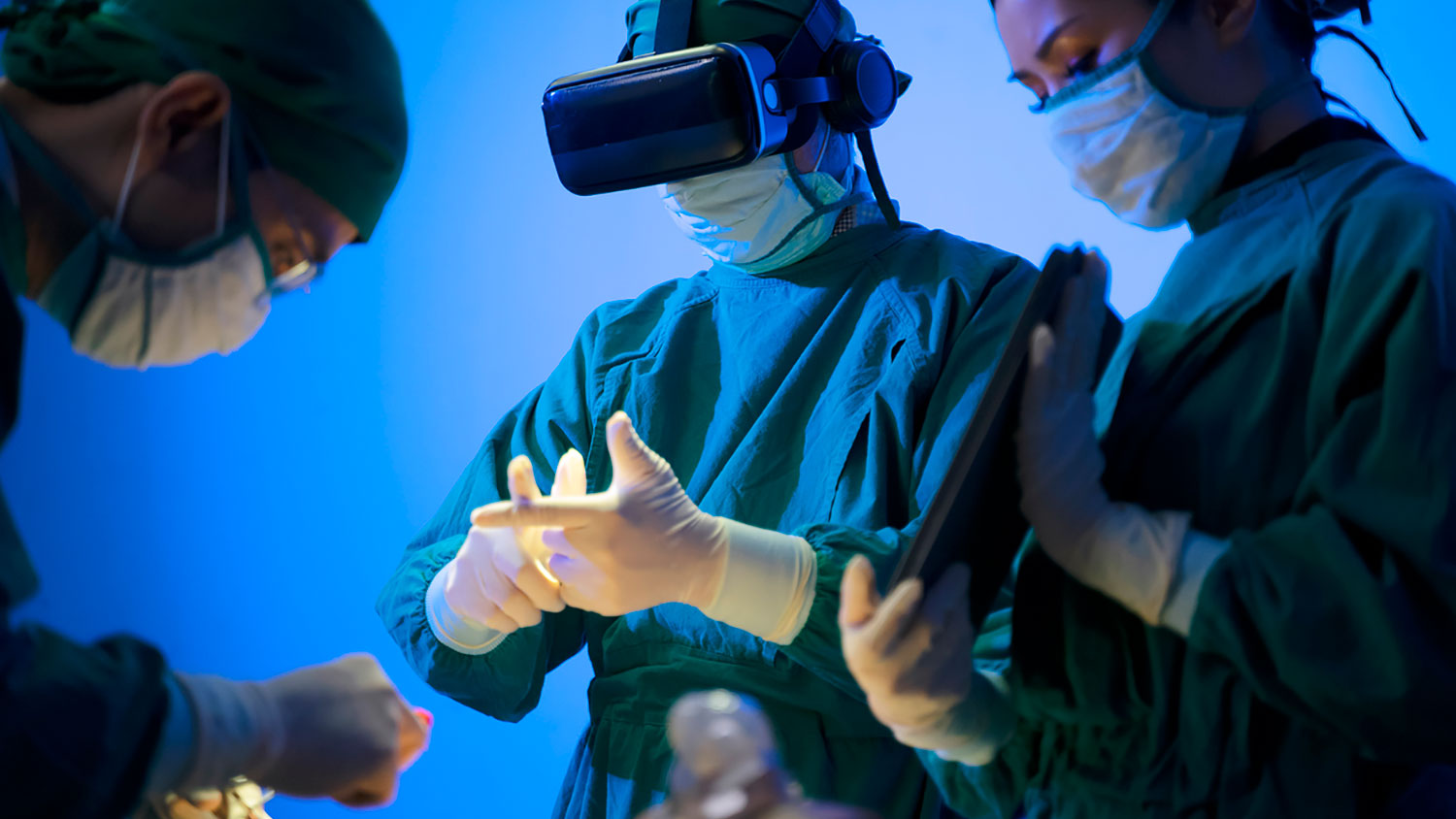
As an experienced Contract Development Manufacturer Organisation for over 30 years…
Partnering with a CDMO gives you access to specialised expertise, state-of-the-art technology, and a streamlined manufacturing process. Together, we are dedicated to pushing the boundaries of innovation, ensuring the highest quality standards, and ultimately, improving patient outcomes. Our ability and resources can cater to customer’s desired production quantity. We have the necessary space and equipment to increase production, yet also have the ability to downsize when needed. We are able to fully support your innovation journey from its seed concept, all the way till prototyping and mass manufacturing.
Explore our website to learn more about how this strategic alliance is shaping the future of healthcare.
Contract Development
CDMOs assist MedTech companies in the development of new medical devices. This can include services such as product development, process development, analytical method development, testing and clinical trial support.
Contract Manufacturing
Equipped with manufacturing capabilities to produce low or high-mix medical products on a contract basis. This includes the production of plastic molded parts, non-plastic components, PCBA, and even reagent. Racer have state-of-the-art facilities that can comply with regulatory standards, such as FDA and ISO 13485 QMS Standards.